MRL Pesticides
Below is a decision tree explaining when in the context of Article 19 of Regulation 178/2002 an exceedance of the maximum residue limit (MRL) for pesticide residues must or must not be reported to the Dutch Food Safety Authority, taking into account the measurement uncertainty (to be found on the certificate of analysis, max. 50%). MRLs can be found in the pesticide database of the European Commission ((EU Pesticides Database (europa.eu))
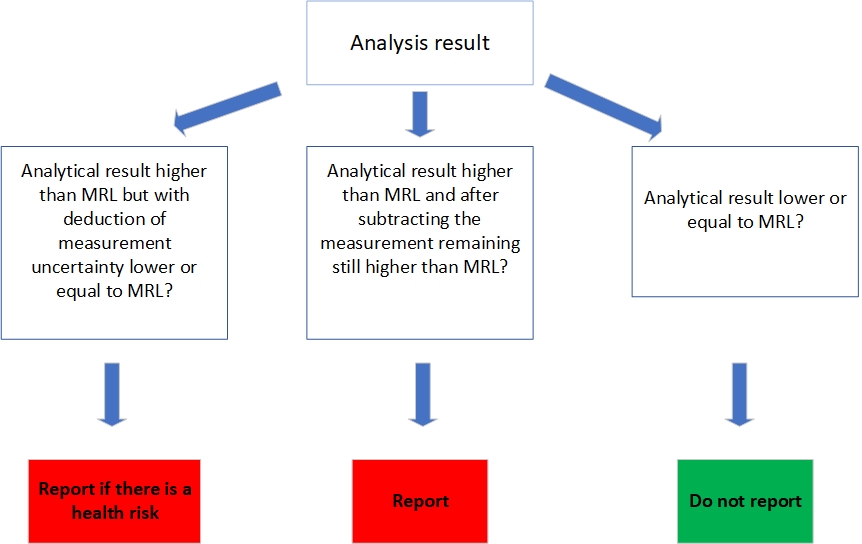
Decision tree MRL
There is a health risk is determined by testing against the health-related limit value:- the Acute Reference Dose (ARfD)* of the active substance, or, if there is none, the Acceptable Daily Intake (ADI)** of the active substance. If both health-related limit values are not exceeded, it has yet to be determined whether the substance is a CMR (carcinogenic – mutagenic – reproduction toxic) substance. When testing whether there is a health risk, the measurement uncertainty should not be taken into account, and the analysis result should therefore be used. For the practical elaboration of the above, see the decision tree ‘assessment of health risk’ on the next page.
ARfD
*The ARfD is the amount of a substance that you can get in 1x without there being a health risk. The ARfD is therefore a health-related limit value for acute intake and not a product standard. The ARfD can be found in the pesticide database.
ADI
**The ADI is the health-related limit value for long-term exposure. This is the amount of a substance that you can ingest daily for a long period of time without there being a health risk. The ADI can also be found in the MRL pesticide database.
Decision tree health risk assessment
Below is which steps must be taken when assessing whether there is a health risk and when it should or should not be reported. The Pesticide database is used for this and the EFSA Primo model (Pesticide residue intake model 3.1). In the decision tree below are the steps that must be followed to properly carry out the test whether there is a health risk. On the last page, a number of examples are given.
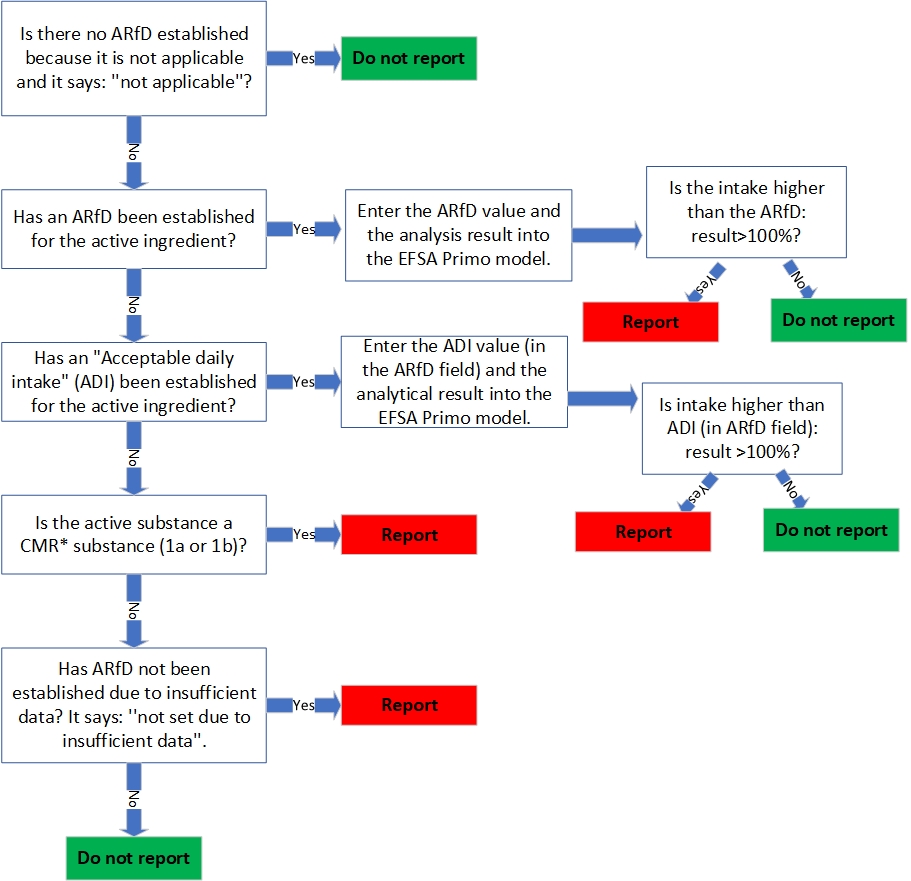
*CMR substances: carcinogenic, mutagenic and reproductively toxic substances. If a substance is a CMR substance, this is indicated in the EU Pesticides Database for the active substance in question under the heading ‘classification’. Only if the indication 1a and/or 1b must be reported.
Examples
Intended in the event that the analytical result for pesticide residues is higher than the MRL, but with deduction of measurement, uncertainty falls below or equal to the MRL.
| Case | Example | Reference Values | Source | Report status |
| Has no ARfD been established because it does not apply and does it say: “not applicable”? >> yes | fluodioxinil | ADI : 0.37 mg/kg bw/dayARfD : N/A | DR07/76DR07/76 | Do not report |
| Has an acute reference dose (ARfD) been established for the active substance? >> yes | metalaxyl | ADI : 0.02 mg/kg bw/dayARfD : 0.5 mg/kg bw | 2010/28/EU 2010/28/EU | Report if ingestion in PRIMO >100% of the ARfD |
| Has an Acceptable daily intake (ADI) been established but no ARfD? >> yes | pyrazophos | ADI : 0.004 mg/kg bw/dayARfD : | IMPR 1993 | Report if ingestion in PRIMO >100% of the ARfD |
| Is the active substance a CMR* substance (1a or 1b)? >> yes | anthraquinone (Classification Reg. 1272/2008-Carc 1B-H350) | ADI :ARfD : | Reporting (See Classification) | |
| Has no ARfD been established due to insufficient data? It says: “not set due to insufficient data” >> yes | chlorpyrifos | ADI : Not set due insufficient dataARfD : Not set due insufficient data | Reg (EU) 2020/18 Reg (EU) 2020/18 | Report |
Related articles to MRL exceeded? What should the food company do?
Many customers and visitors to this page 'MRL exceeded? What should the food company do?' also viewed the articles and manuals listed below:



